ER‑100 is emerging as one of the most closely watched experimental therapies in the field of aging and regenerative medicine. Developed by Life Biosciences, the treatment represents a bold attempt to harness partial epigenetic reprogramming—a technique that aims to restore youthful cellular function without erasing a cell’s identity. Recent regulatory milestones and scientific interest have pushed ER‑100 into the spotlight as it enters first‑in‑human clinical trials.
What ER‑100 Is Designed to Do
ER‑100 is an investigational gene therapy that uses adeno‑associated virus (AAV) vectors to deliver three transcription factors—Oct4, Sox2, and Klf4, collectively known as OSK. These factors are central to the concept of partial epigenetic reprogramming, which seeks to reverse age‑related epigenetic changes that accumulate over time. By nudging cells toward a more youthful state, ER‑100 aims to restore function without pushing them into full dedifferentiation, a risk associated with complete reprogramming.

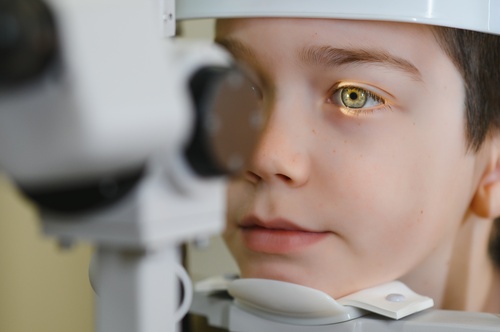
The therapy is being developed primarily for optic neuropathies, including glaucoma and non‑arteritic anterior ischemic optic neuropathy (NAION). These conditions involve damage to retinal ganglion cells, which do not naturally regenerate. ER‑100’s goal is to preserve or restore these cells’ function by rejuvenating their epigenetic landscape.
Regulatory Progress and Clinical Trials
In January 2026, the U.S. Food and Drug Administration cleared Life Biosciences’ Investigational New Drug (IND) application for ER‑100, marking the first time a cellular rejuvenation therapy based on partial epigenetic reprogramming has been approved to enter human trials. This milestone allows the company to begin evaluating ER‑100’s safety and potential therapeutic effects in patients with optic neuropathies.
The Phase I clinical trial will recruit individuals diagnosed with NAION and open‑angle glaucoma. Its objectives include assessing immune responses, tolerability, safety, and changes in visual function. This early‑stage study is not designed to prove efficacy but to establish whether ER‑100 can be administered safely while offering preliminary insights into its impact on vision.
Scientific and Cultural Context

ER‑100 arrives at a moment when interest in reversing aspects of human aging is surging. High‑profile figures in technology and science have publicly speculated that aging may be more malleable than once believed. Researchers like Harvard’s David Sinclair have long argued that epigenetic changes are a primary driver of aging and that reprogramming technologies could restore youthful function. This broader cultural and scientific momentum has helped propel ER‑100 into public conversation.
At the same time, the therapy has attracted scrutiny. Some scientists caution that reprogramming technologies carry risks, including potential tumor formation or unintended cellular changes. Others note that the field is still in its infancy, and long‑term effects remain unknown. Nonetheless, the launch of human trials marks a significant step forward, signaling that regulators are willing to explore the safety of these approaches under controlled conditions.
Looking Ahead
ER‑100 represents a convergence of cutting‑edge gene therapy, aging biology, and clinical need. If successful, it could open the door to new treatments for vision loss and potentially other age‑related diseases. While many questions remain, the therapy’s entry into human trials underscores a growing confidence that partial epigenetic reprogramming may one day become a practical tool for restoring function in aging tissues.